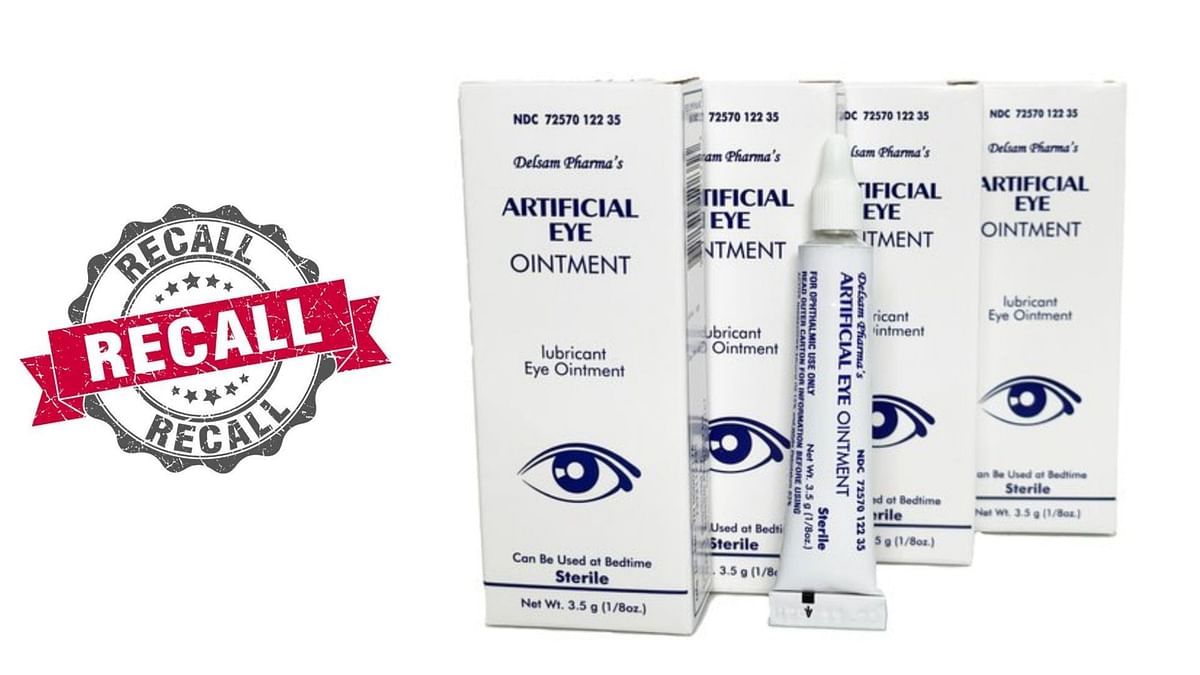
Recall On Eye Drops 2025 - What eye drops are contaminated? FDA expands warning recall list, Explanation of identified safety issue. And centered around eye ointment products sold at. Another Death, More Cases of Vision Loss Linked to Tainted Eye Drops, Fda raises concerns over potential infection risk. Announced by the fda on february 26, 2025, the nationwide recall was issued by brassica pharma pvt.
What eye drops are contaminated? FDA expands warning recall list, Explanation of identified safety issue. And centered around eye ointment products sold at.
:quality(80)/cloudfront-us-east-1.images.arcpublishing.com/semana/OKQNZHBHFVEF3ALWFQXNT2GNO4.jpeg)
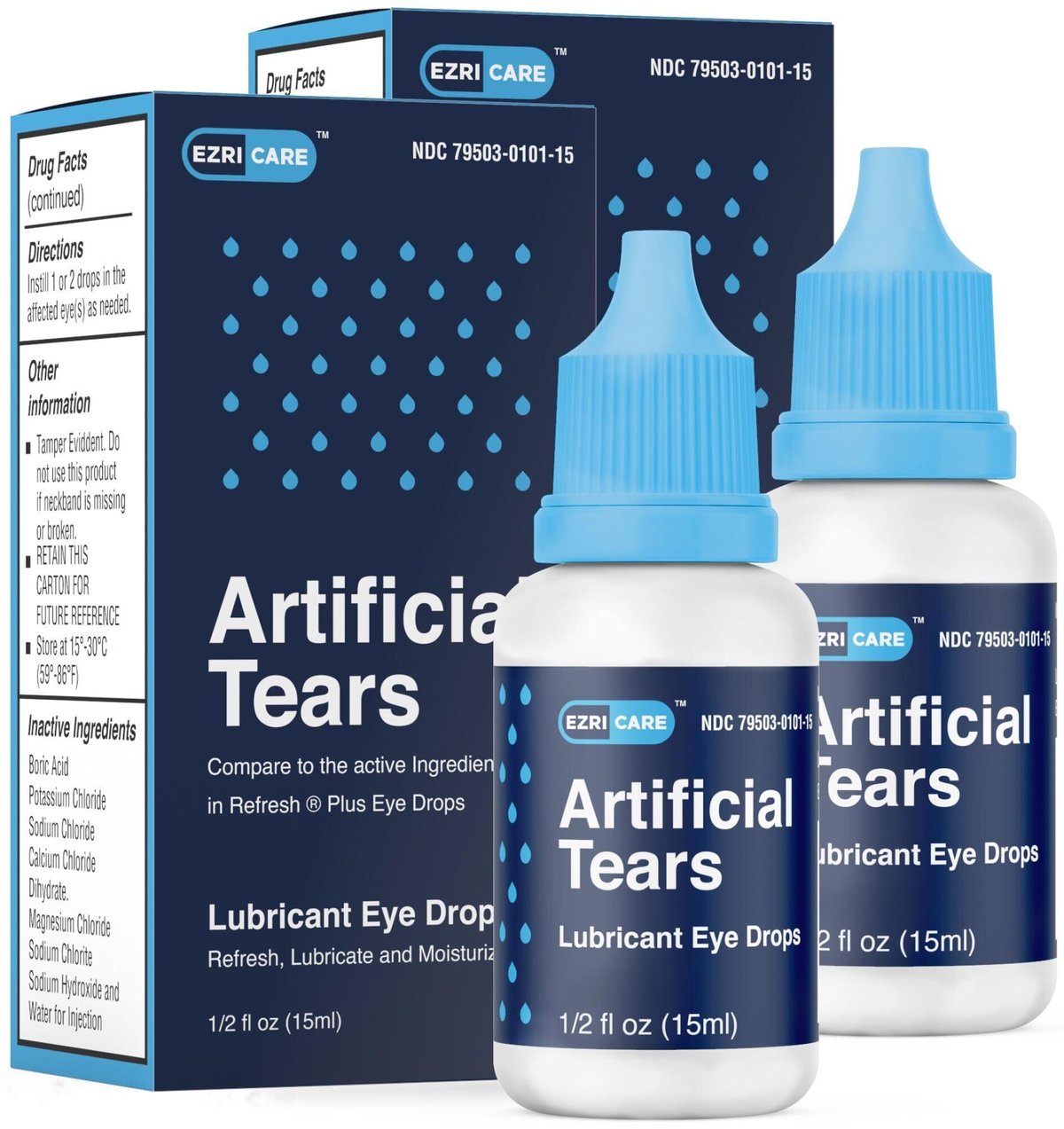
A full list of potentially contaminated artificial tears.

Buy Refresh Tears Lubricant eye drops 4×15 ml, 1×5 ml Online at, Janice haney carr/cdc via ap. In some cases, certain products may be linked to vision loss and other.
Recall On Eye Drops 2025. A full list of potentially contaminated artificial tears. Explanation of identified safety issue.

EzriCare Eye Drops Lawsuit — Lawsuit Information Center, Fda is recommending that you discard these products immediately. Over the course of 2023 and 2025, a long list of artificial tear products have been recalled.

Lágrimas artificiales cuándo y cómo deben utilizarse adecuadamente, New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned. See below for the eye ointments affected by the recall;

More Eye Drops, Ointment Added to Nationwide RecallSee the List, Apotex, a canadian pharmaceutical company, recalled prescription eyedrops in march after some bottle caps developed cracks, which could compromise. New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned.
Your eyedrops could be filled with filthy bacteria.
Your eyedrops could be filled with filthy bacteria. The fda says ‘ultimately industry is responsible for the quality of their products’.

Eye drop recall 2023 FDA finds sterilization issues at EzriCare, Global pharma healthcare recalled artificial tears lubricant eye drops, distributed by ezricare and delsam pharma, due to possible bacterial contamination that. In 2023, multiple eye drops were recalled.

Several eye drops and ointment sold at Walgreens and Walmart recalled, New york cnn — eye ointments sold at cvs and walmart may not actually be sterile, a recall posted by the us food and drug administration warned. Eye drops recalled after cdc links them to vision loss, 1 death.